Budesonide
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Pulmicort, Rhinocort, Entocort, others |
| Other names | BUD |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a608007 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, nasal, tracheal, rectal, inhalation |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 10-20% (first pass effect) |
| Protein binding | 85-90% |
| Metabolism | Liver CYP3A4 |
| Elimination half-life | 2.0-3.6 hours |
| Excretion | Urine, feces |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.051.927 |
| Chemical and physical data | |
| Formula | C25H34O6 |
| Molar mass | 430.541 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Budesonide, sold under the brand name Pulmicort, among others, is a steroid medication.[8] It is available as an inhaler, nebulization solution, pill, nasal spray, and rectal forms.[8][9] The inhaled form is used in the long-term management of asthma and chronic obstructive pulmonary disease (COPD).[8][10][11] The nasal spray is used for allergic rhinitis and nasal polyps.[9][12] Modified-release pills or capsules and rectal forms may be used for inflammatory bowel disease including Crohn's disease, ulcerative colitis, and microscopic colitis.[13][14][15]
Common side effects with the inhaled form include respiratory infections, cough, and headaches.[16] Common side effects with the pills include feeling tired, vomiting, and joint pains.[16] Serious side effects include an increased risk of infection, loss of bone strength, and cataracts.[16] Long-term use of the pill form may cause adrenal insufficiency.[16] Stopping the pills suddenly following long-term use may therefore be dangerous.[16] The inhaled form is generally safe in pregnancy.[16] Budesonide chiefly acts as a glucocorticoid.[16]
Budesonide was initially patented in 1973.[17] Commercial use as an asthma medication began in 1981.[18] It is on the World Health Organization's List of Essential Medicines.[19] Some forms are available as a generic medication.[20][21] In 2021, it was the 185th most commonly prescribed medication in the United States, with more than 2 million prescriptions.[22][23]
Medical uses[edit]
Asthma[edit]
Budesonide is given by metered-dose inhaler or nebulizer for maintenance and prophylactic treatment of asthma, including patients who require oral corticosteroids and those who may benefit from a systemic dose reduction.[24]
Inflammatory bowel disease[edit]
Formulations of delayed-release budesonide are an effective treatment for mild-to-moderately active Crohn's disease involving the ileum and/or ascending colon.[25] A Cochrane review found evidence for up to three months (but not longer) of maintenance of remission in Crohn's disease.[26]
Budesonide assists in the induction of remission in people with active ulcerative colitis.[27]
Budesonide is highly effective and recommended as the drug of choice in microscopic colitis, for induction and maintenance of remission, and for both the lymphocytic colitis and collagenous colitis forms.[28][29]
Allergic rhinitis[edit]
Budesonide in the form of nasal sprays is a treatment for allergic rhinitis.[30]
Eosinophilic esophagitis[edit]
Topical budesonide has considerable effects in eosinophilic esophagitis.[31] For this use, it is formulated as a tablet that disperses in the mouth, and sold under the brand name Jorveza.[32]
Berger's disease[edit]
Budesonide (Tarpeyo (US); Kinpeygo (EU, UK)) is indicated to reduce proteinuria (increased protein levels in the urine) in adults with primary immunoglobulin A (IgA) nephropathy (Berger's disease) at risk of rapid disease progression.[3][33][7]
Side effects[edit]
Nasal budesonide inhalers have been associated with a number of side effects.[34][35] These include nose irritation or burning, bleeding or sores in the nose, lightheadedness, upset stomach, cough, hoarseness, dry mouth, rash, sore throat, bad taste in mouth, change in mucus, and blurred vision.[36] Other symptoms which should be reported immediately include difficulty in breathing, swelling of the face, white patches in the throat, mouth, or nose, irregular menstrual periods, severe acne, and on rare occasions, behavioral changes (mostly affecting children).[34]
Overdose[edit]
Acute toxicity from an overdose of Budesonide is significantly more rare than an overdosing of budesonide over a prolonged period of therapy, however both can can cause systemic toxicity that manifests as hypercortisolism.[37] Symptoms of an overdose include more specific symptoms such as darkening and thinning of the skin, changes in body fat around the face, neck, back, and waist, increased acne or facial hair, menstrual problems, impotence, or loss of interest in sex, as well as some less specific symptoms such as diarrhea, dizziness, loss of appetite, mental depression, nausea, skin rash, unusual tiredness or weakness, or vomiting.[38][39]
Contraindications[edit]
Budesonide is contraindicated in people with:
- Known hypersensitivity to budesonide or any component of the formulation.[40][41]
- Status asthmaticus or other acute episodes of asthma which would require intensive, immediate measures.[40][42]
In Canada, there are additional contraindications labeled for Budesonide for people with:
- Systemic or local bacterial, fungal and viral infections.[40]
- Active or quiescent pulmonary tuberculosis.[40]
Interactions[edit]
Budesonide is mainly metabolized in the liver by the enzyme CYP34A.[43][44] Drugs that are CYP3A4 inhibitors such as ketoconazole, clarithromycin, ritonavir, and nefazodone, among many others, may inhibit the metabolism of Budesonide, prolonging its elimination and leading to possible increased rates of corticosteroid adverse effects due to unwanted drug accumulation.[43][45] Grapefruit is also a potent inhibitor of CYP3A4, and therefore its consumption is not recommended while on budesonide treatment.[43][46]
Pharmacology[edit]
Mechanism of action[edit]
Budesonide is an agonist of glucocorticoid receptors. Among its effects are:
- Controls the rate of protein synthesis.[47]
- Depresses the migration of polymorphonuclear leukocytes and fibroblasts.[48]
- Reverses capillary permeability and lysosomal stabilization at the cellular level to prevent or control inflammation.[47]
- Has a potent glucocorticoid activity and weak mineralocorticoid activity.[49]
Pharmacokinetics[edit]
Different pharmacokinetic proprieties can be seen in the absorption of budesonide depending on how it is formulated. When taken as an extended-release oral capsule, budesonide has an oral bioavailability of 9–21% and reaches peak plasma concentrations (Cmax) within 2–8 hours.[50] A high fat meal when taken with the capsule can lengthen the time it takes to reach Cmax by another 2.3 hours, but will not have any other affects on the pharmacokinetics properties of budesonide.[51] When inhaled through an metered dose inhaler, 34% of budesonide is deposited in the lung with a bioavailability of 39% and reaches Cmax within 10 minutes.[51][52] When nebulized, budesonide has an bioavailability of 6% and reaches Cmax within 1–3 hours.[52][51] When formulated as a rectal foam, budesonide has an bioavailability of 3% to 27% and reaches Cmax around 1.5 hours.[53]
The plasma protein binding of budesonide is around 85-90%, with an apparent volume of distribution of 2.2-3.9L/kg.[51][50] Budesonide is 80-90% metabolized at first pass in the liver by the hepatocytic cytochrome P450 isoenzyme 3A4 (CYP3A4) into two metabolites: 16 alpha-hydroxyprednisolone and 6 beta-hydroxybudesonide. Both of these metabolites have a negligible glucocorticoid activity of less than 1% compared to the parent compound budesonide.[51] 60% of budesonide is excreted is through the urine as its metabolites, no unchanged budesonide is detectable in urine.[51] The average elimination half-life in plasma is between 2-3.6 hours.[51]
Chemistry[edit]
Budesonide, also known as 11β,21-dihydroxy-16α,17α-(butylidenebis(oxy))pregna-1,4-diene-3,20-dione, is a synthetic pregnane steroid and non-halogenated cyclic ketal corticosteroid.[54][55] It is the C16α hydroxyl, C16α,17α cyclic ketal with butyraldehyde derivative of prednisolone (11β,17α,21-trihydroxypregna-1,4-diene-3,20-dione).[54][55]
Stereoisomerism[edit]
| Budesonide (2 stereoisomers) | |
|---|---|
 (22R)-configuration |
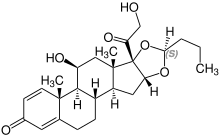 (22S)-configuration |
Society and culture[edit]

Brand names[edit]
Budesonide is a drug that is marketed under various brand and generic names internationally, some notable examples for each formulation are listed here;
Inhalation: Pulmicort; Pulmicort Flexhaler, Pulmicort Nebuamp, Pulmicort Turbuhaler, TARO-Budesonide, TEVA-Budesonide, Novolizer budesonid meda, Budenova.[56][57]
Systemic (oral pills): Tarpeyo, Uceris, Eohilia, Cortiment, Entocort Jorveza.[58][57]
Nasal: MYLAN-Budesonide AQ, Rhinocort Aqua, Formancis.[59][57]
Topical: Entocort, Uceris, Budenofalk.[60][57]
Economics[edit]
In 2019, generic budesonide was listed as being involved in Teva's price fixing scheme in the United States.[61]
Legal status[edit]
In May 2022, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a conditional marketing authorization for the medicinal product Kinpeygo, intended for the treatment of primary immunoglobulin A nephropathy.[62] The applicant for this medicinal product is Calliditas Therapeutics AB.[62] Kinpeygo is a hybrid medicine of Entocort which has been authorised in the EU since 2 April 1992.[62] Kinpeygo contains the same active substance as Entocort but has a different formulation and a different indication.[62] Kinpeygo was approved for medical use in the European Union in July 2022.[7][63]
Research[edit]
COVID-19[edit]
Budesonide was recommended in April 2021 by the UK's NHS to treat COVID-19 on a case-by-case basis for those aged 50 years of age and older.[64] After a University of Oxford research team found in a trial with 1,700 patients that budesonide could benefit many people over 50 with COVID-19 symptoms, it was recommended from 12 April 2021, by the National Health Service in the UK for general practitioners (GPs) to treat COVID-19 on a case-by-case basis.[65][66] Results of a large-scale trial published in August 2021 suggest that inhaled budesonide improves the time of recovery and people's well-being during the recovery process.[67][68] Inhalational budesonide was added to the recommended treatment for cases of COVID-19 in India in April 2021.[69][70] The NIH recommendation was withdrawn in December 2021 citing the need for more research.[71][72]
References[edit]
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ "Regulatory Decision Summary - Uceris". Health Canada. 23 October 2014. Archived from the original on 5 June 2022. Retrieved 4 June 2022.
- ^ a b "Tarpeyo- budesonide capsule, delayed release". DailyMed. Archived from the original on 25 December 2021. Retrieved 24 December 2021.
- ^ "Pulmicort Flexhaler- budesonide aerosol, powder". DailyMed. Archived from the original on 19 September 2021. Retrieved 24 December 2021.
- ^ "Eohilia- budesonide suspension". DailyMed. 9 February 2024. Archived from the original on 26 February 2024. Retrieved 26 February 2024.
- ^ "Jorveza EPAR". European Medicines Agency (EMA). 17 September 2018. Archived from the original on 4 March 2023. Retrieved 3 March 2023.
- ^ a b c "Kinpeygo EPAR". European Medicines Agency (EMA). 17 May 2022. Archived from the original on 4 March 2023. Retrieved 3 March 2023.
- ^ a b c "Budesonide". The American Society of Health-System Pharmacists. Archived from the original on 28 November 2015. Retrieved 2 December 2015.
- ^ a b "Budesonide eent". The American Society of Health-System Pharmacists. Archived from the original on 8 December 2015. Retrieved 2 December 2015.
- ^ De Coster DA, Jones M (2014). "Tailoring of corticosteroids in COPD management". Current Respiratory Care Reports. 3 (3): 121–132. doi:10.1007/s13665-014-0084-2. PMC 4113685. PMID 25089228.
- ^ Christophi GP, Rengarajan A, Ciorba MA (2016). "Rectal budesonide and mesalamine formulations in active ulcerative proctosigmoiditis: efficacy, tolerance, and treatment approach". Clinical and Experimental Gastroenterology. 9: 125–30. doi:10.2147/CEG.S80237. PMC 4876845. PMID 27274301.
- ^ Rudmik L, Schlosser RJ, Smith TL, Soler ZM (July 2012). "Impact of topical nasal steroid therapy on symptoms of nasal polyposis: a meta-analysis". The Laryngoscope. 122 (7): 1431–7. doi:10.1002/lary.23259. PMID 22410935. S2CID 25637461.
- ^ Silverman J, Otley A (July 2011). "Budesonide in the treatment of inflammatory bowel disease". Expert Review of Clinical Immunology. 7 (4): 419–28. doi:10.1586/eci.11.34. PMID 21790284. S2CID 32892611.
- ^ Pardi DS, Tremaine WJ, Carrasco-Labra A (January 2016). "American Gastroenterological Association Institute Technical Review on the Medical Management of Microscopic Colitis". Gastroenterology. 150 (1): 247–274.e11. doi:10.1053/j.gastro.2015.11.006. PMID 26584602.
- ^ British national formulary: BNF 58 (58 ed.). British Medical Association. 2009. pp. 56–57. ISBN 9780857111562.
- ^ a b c d e f g "Budesonide". The American Society of Health-System Pharmacists. Archived from the original on 28 November 2015. Retrieved 2 December 2015.
- ^ Domeij B (2000). Pharmaceutical patents in Europe. The Hague: Kluwer Law International. p. 278. ISBN 9789041113481. Archived from the original on 8 December 2015.
- ^ Hamley P (2015). Small Molecule Medicinal Chemistry: Strategies and Technologies. John Wiley & Sons. p. 390. ISBN 9781118771693. Archived from the original on 8 December 2015.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ Hamilton R (2015). Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. p. 451. ISBN 9781284057560.
- ^ "First Generic Drug Approvals 2023". U.S. Food and Drug Administration (FDA). 30 May 2023. Archived from the original on 30 June 2023. Retrieved 30 June 2023.
- ^ "The Top 300 of 2021". ClinCalc. Archived from the original on 15 January 2024. Retrieved 14 January 2024.
- ^ "Budesonide - Drug Usage Statistics". ClinCalc. Archived from the original on 29 April 2017. Retrieved 14 January 2024.
- ^ Global Strategy for Asthma Management and Prevention, Global Initiative for Asthma (GiNA) 2011. Available at https://www.ginasthma.org Archived 14 October 2013 at the Wayback Machine
- ^ Lichtenstein GR, Hanauer SB, Sandborn WJ (2009). "Management of Crohn's Disease in Adults". Am J Gastroenterol. 104 (2): 465–83. doi:10.1038/ajg.2008.168. PMID 19174807. S2CID 10176441.
- ^ Kuenzig ME, Rezaie A, Seow CH, Otley AR, Steinhart AH, Griffiths AM, et al. (2014). "Budesonide for maintenance of remission in Crohn's disease". Cochrane Database Syst Rev. 8 (8): CD002913. doi:10.1002/14651858.CD002913.pub3. PMC 7133546. PMID 25141071.
- ^ Habal FM, Huang VW (2012). "Review Article: A Decision-Making Algorithm For the Management of Pregnancy in the Inflammatory Bowel Disease Patient". Aliment Pharmacol Ther. 35 (5): 501–15. doi:10.1111/j.1365-2036.2011.04967.x. PMID 22221203. S2CID 34662981.
- ^ Pardi DS, Tremaine WJ, Carrasco-Labra A (January 2016). "American Gastroenterological Association Institute Technical Review on the Medical Management of Microscopic Colitis". Gastroenterology. 150 (1): 247–274.e11. doi:10.1053/j.gastro.2015.11.006. PMID 26584602.
- ^ Miehlke S, Guagnozzi D, Zabana Y, Tontini GE, Kanstrup Fiehn AM, Wildt S, et al. (February 2021). "European guidelines on microscopic colitis: United European Gastroenterology and European Microscopic Colitis Group statements and recommendations". United European Gastroenterology Journal. 9 (1): 13–37. doi:10.1177/2050640620951905. PMC 8259259. PMID 33619914.
- ^ Stanaland BE (April 2004). "Once-daily budesonide aqueous nasal spray for allergic rhinitis: a review". Clinical Therapeutics. 26 (4): 473–92. doi:10.1016/s0149-2918(04)90050-1. PMID 15189745.
- ^ Rawla P, Sunkara T, Thandra KC, Gaduputi V (December 2018). "Efficacy and Safety of Budesonide in the Treatment of Eosinophilic Esophagitis: Updated Systematic Review and Meta-Analysis of Randomized and Non-Randomized Studies". Drugs in R&D. 18 (4): 259–269. doi:10.1007/s40268-018-0253-9. PMC 6277325. PMID 30387081.
- ^ UK Drug Information
- ^ "FDA approves first drug to decrease urine protein in IgA nephropathy, a rare kidney disease". U.S. Food and Drug Administration (FDA). 17 December 2021. Archived from the original on 19 December 2021. Retrieved 18 December 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ a b "GENERIC NAME: BUDESONIDE - NASAL AEROSOL INHALER (byou-DESS-oh-nide)". eMedicineHealth. Archived from the original on 6 November 2008.
- ^ "What are the possible side effects of budesonide nasal (Childrens Rhinocort Allergy, Rhinocort Allergy, Rhinocort Aqua)?". eMedicineHealth. Archived from the original on 6 August 2020. Retrieved 25 August 2020.
- ^ "Budesonide: CMDh scientific conclusions and grounds for variation, amendments to the product information and timetable for the implementation - PSUSA/00000449/201604" (PDF). European Medicines Agency (EMA). 10 March 2017. Archived (PDF) from the original on 8 September 2017. Retrieved 19 May 2017.
- ^ Kalola UK, Ambati S (2024), "Budesonide", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 33085348, retrieved 28 April 2024
- ^ "Budesonide (Oral Route) Precautions - Mayo Clinic". www.mayoclinic.org. Retrieved 3 May 2024.
- ^ "Budesonide inhalation Uses, Side Effects & Warnings". Drugs.com. Retrieved 3 May 2024.
- ^ a b c d Kalola UK, Ambati S (2024), "Budesonide", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 33085348, retrieved 28 April 2024
- ^ Todd GR (1 December 2002). "Survey of adrenal crisis associated with inhaled corticosteroids in the United Kingdom". Archives of Disease in Childhood. 87 (6): 457–461. doi:10.1136/adc.87.6.457. PMC 1755820. PMID 12456538.
- ^ Todd GR, Acerini CL, Buck JJ, Murphy NP, Ross-Russell R, Warner JT, et al. (2002). "Acute Adrenal Crisis in Asthmatics Treated With High-Dose Fluticasone Propionate". Eur Respir J. 19 (6): 1207–9. doi:10.1183/09031936.02.00274402. PMID 12108877.
- ^ a b c Kalola UK, Ambati S (2024), "Budesonide", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 33085348, retrieved 28 April 2024
- ^ "Budesonide (Oral Inhalation)". Lexi-Drugs. UpToDate Lexidrug. UpToDate Inc. 28 April 2024.
- ^ Ajimura CM, Jagan N, Morrow LE, Malesker MA (December 2018). "Drug Interactions With Oral Inhaled Medications". Journal of Pharmacy Technology. 34 (6): 273–280. doi:10.1177/8755122518788809. ISSN 8755-1225. PMC 6231282. PMID 34861014.
- ^ Baily DG (2010). "Grapefruit and Other Fruit Juices Interactions with Medicines". In Boullata JI, Armenti VT (eds.). Handbook of drug-nutrient interactions (2nd ed.). New York, NY: Humana Press. p. 282. ISBN 978-1-60327-362-6.
- ^ a b Kizior RJ, Hodgson BB (21 February 2018). Saunders Nursing Drug Handbook 2019 E-Book. Elsevier Health Sciences. p. 160. ISBN 978-0-323-61257-9. Archived from the original on 30 April 2024. Retrieved 5 October 2020.
- ^ Skidmore-Roth L, Richardson F (9 July 2020). Mosby's Canadian Nursing Drug Reference - E-Book. Elsevier Health Sciences. p. 187. ISBN 978-1-77172-084-7. Archived from the original on 11 March 2024. Retrieved 5 October 2020.
- ^ Gavins F, Flower RJ (1 January 2008), Enna SJ, Bylund DB (eds.), "Budesonide", xPharm: The Comprehensive Pharmacology Reference, New York: Elsevier, pp. 1–5, ISBN 978-0-08-055232-3, retrieved 3 May 2024
- ^ a b "Budesonide (Systemic)". Lexi-Drugs. UpToDate Lexidrug. UpToDate Inc. 28 April 2024.
- ^ a b c d e f g "Budesonide". go.drugbank.com. Retrieved 28 April 2024.
- ^ a b "Budesonide (Oral Inhalation)". Lexi-Drugs. UpToDate Lexidrug. UpToDate Inc. 28 April 2024.
- ^ "Budesonide (Topical)". Lexi-Drugs. UpToDate Lexidrug. UpToDate Inc. 28 April 2024.
- ^ a b Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 186, 1011. ISBN 978-1-4757-2085-3. Archived from the original on 8 September 2017.
- ^ a b Lemke TL, Williams DA (2008). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 1253–. ISBN 978-0-7817-6879-5. Archived from the original on 8 September 2017.
- ^ "Budesonide (Oral Inhalation)". Lexi-Drugs. UpToDate Lexidrug. UpToDate Inc. 28 April 2024.
- ^ a b c d "Budesonide". go.drugbank.com. Retrieved 28 April 2024.
- ^ "Budesonide (Systemic)". Lexi-Drugs. UpToDate Lexidrug. UpToDate Inc. 28 April 2024.
- ^ "Budesonide (Nasal)". Lexi-Drugs. UpToDate Lexidrug. UpToDate Inc. 28 April 2024.
- ^ "Budesonide (Topical)". Lexi-Drugs. UpToDate Lexidrug. UpToDate Inc. 28 April 2024.
- ^ Murphy H (11 May 2019). "Teva and Other Generic Drugmakers Inflated Prices Up to 1,000%, State Prosecutors Say". The New York Times. Archived from the original on 30 May 2020. Retrieved 27 May 2020.
- ^ a b c d "Kinpeygo: Pending EC decision". European Medicines Agency (EMA). 20 May 2022. Archived from the original on 20 May 2022. Retrieved 20 May 2022. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ "Kinpeygo Product information". Union Register of medicinal products. Archived from the original on 4 March 2023. Retrieved 3 March 2023.
- ^ "COVID-19 Therapeutic Alert - Inhaled Budesonide for Adults (50 Years and Over) with COVID-19". Central Alerting System. 12 April 2021. Archived from the original on 12 April 2021. Retrieved 16 April 2021.
- ^ Roxby P (12 April 2021). "Covid: Asthma drug 'speeds up recovery at home'". BBC News. Archived from the original on 12 April 2021. Retrieved 12 April 2021.
- ^ Blakely R (12 April 2021). "Asthma drug Budesonide speeds up Covid recovery times". The Times. ISSN 0140-0460. Archived from the original on 6 November 2021. Retrieved 13 April 2021.
- ^ "Platform trial rules out treatments for COVID-19". NIHR Evidence (Plain English summary). 31 May 2022. doi:10.3310/nihrevidence_50873. Archived from the original on 1 June 2022. Retrieved 1 June 2022.
- ^ Yu LM, Bafadhel M, Dorward J, Hayward G, Saville BR, Gbinigie O, et al. (September 2021). "Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial". Lancet. 398 (10303): 843–855. doi:10.1016/S0140-6736(21)01744-X. PMC 8354567. PMID 34388395.
- ^ "New guidelines prescribe inhaled steroid 'Budesonide', antiparasitic drug 'Ivermectin' for mild Covid cases". The New Indian Express. 29 April 2021. Archived from the original on 6 November 2021. Retrieved 11 July 2021.
- ^ "Clinical Management Protocol for Covid-19 (in Adults)" (PDF). Ministry of Health and Family Welfare (India). 24 May 2021. Archived (PDF) from the original on 5 December 2021. Retrieved 10 July 2021.
- ^ Burns C (15 December 2021). "NICE removes budesonide from recommended COVID-19 treatments". The Pharmaceutical Journal. Archived from the original on 26 June 2022. Retrieved 1 June 2022.
- ^ "Withdrawal of the Recommendation for Consideration of Inhaled Budesonide as a Treatment Option for COVID-19". Central Alerting System. Archived from the original on 30 June 2022. Retrieved 20 May 2022.
External links[edit]
- "Budesonide Nasal Spray". MedlinePlus.
- "Budesonide Oral Inhalation". MedlinePlus.
